How Is the Periodic Law Demonstrated in the Halogens
Also I need help writing the noble gas notation for the electron configuration of each of the following elements and indicate the period in which they belong. Students identified most of the halogens on the periodic table of the elements and.

Tennessine Definition Facts Britannica
Although astatine is radioactive and only has short-lived isotopes it behaves similar to iodine and is often included in the halogen group.
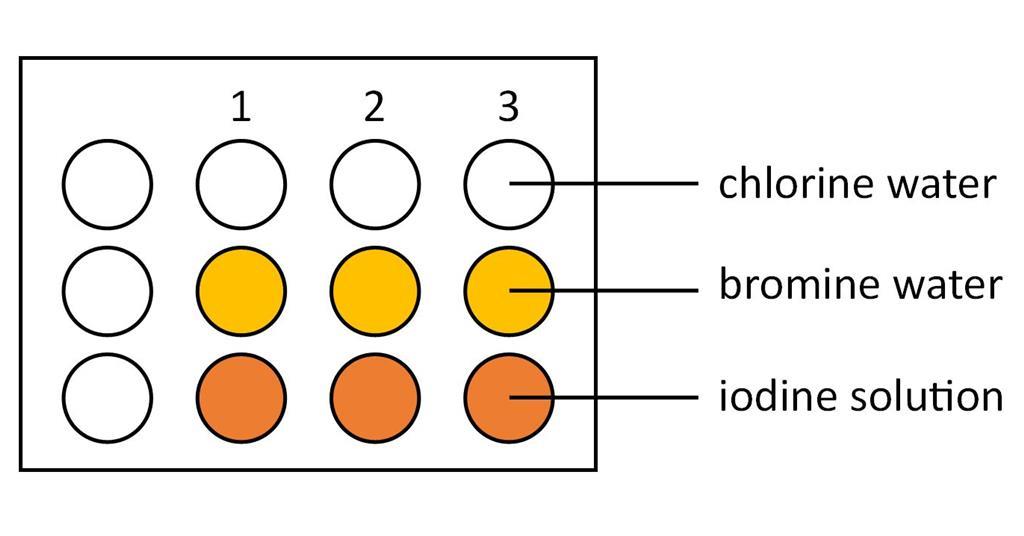
. How is the periodic law demonstrated within the group of the periodic table. Demonstrated a clear understanding of the role acids play in digestion and of why acids can have a positive and negative effect on digestion. All halogens have the same number of valence electrons and similar chemical properties.
Alkali metals are more reactive than alkaline earth metals. Decreasing atomic radius increasing ionization energy it takes more energy to release an electron from an atom increasing electron affinity more energy is released when an atom gains an electron and increasing elecronegativity atoms have an increased attraction for electrons. Like the alkali metals the halogens are extremely reactive.
There are eight main groups of elements numbered 1 2 and 13-18. As every periodic table contains 7 valence electrons and they only need 1 more to complete an outer shell that is why they are extremely reactive. The periodic law demonstrated in halogens by.
These five toxic non-metallic elements make up Group 17 of the periodic table and consist of. Elements in the same group usually have similar properties because they have the same number of electrons in the outermost electron shell. What is the relation between.
A group is any column on the periodic table. How is the periodic law demonstrated within the groups of the periodic table. Why are the noble gases relatively unreactive.
Up to 24 cash back How is the periodic law demonstrated within the groups of the Periodic Table. Groups of elements exhibit similar physical and chemical behavior. How is the periodic law demonstrated in the halogens.
The periodic law arranges the elements. Chemistry 21062019 2000 Renabelle6350. What best describes the law of independent assortment.
Physical and chemical properties of the elements are periodic functions on their atomic numbers. The noble gas elements have practically zero electron affinity because they have full valence electron subshells. The periodic table lists and organizes all known elements.
Halogens Halogens the nonmetals located in group 17. Are you able to identify the alkali metals alkaline earth metals transition metals halogens and noble gases and list three characteristic properties of each family. The halogens are located on the left of the noble gases on the periodic table.
Accurately described several characteristics of these elements. All halogens have the same number of valence electrons and similar chemical properties Explain why. Chemistry 22062019 0230 drivinghydra.
How is the periodic law demonstrated in the halogens. Why do the halogens readily form 1 ions. Because of this phenomenon it is possible to construct a table that.
1 Get Other questions on the subject. And according to the law that recurring patterns of the properties of elements arise when they are arranged in order of increasing atomic number. This means that the properties of the elements are periodic or recurring functions of their atomic numbers.
How is the periodic law demonstrated within the groups of the periodic table. Describe difference between alkali metals and alkaline earth metals. How is the periodic law demonstrated within the groups of the periodic table.
Halogens group 17. The periodic law is. Identify the most- and least-electronegative groups of elements in the periodic table.
I just need to know why. You might be interested. In contrast the halogens readily accept electrons to fill their electron subshells and have high electron affinities.
Why do halogens readily form 1- ions. They all have similar chemical properties and reactivity. The periodic law states that there is a periodic repetition of chemical and physical properties of the elements when they are arranged by increasing.
Fluorine F chlorine Cl bromine Br iodine I and astatine At. They need to gain one electron to be stable. - Most reactive nonmetals.
How is the periodic law demonstrated in the halogens. Using Periodic Law it becomes apparent the alkaline earth elements have a low electron affinity. Which elements are designated as the halogens.
When elements on the periodic table are arranged by atomic number relationships and similarities in properties can be seen. Iknow 2 is d. Demonstrated a connection between atomic mass and.
How is the periodic law demonstrated in halogens How is the periodic law demonstrated in halogens Answers. The halogens are the reactive non-metals in the periodic table it reacts with alkali metals. List three of their characteristic properties of halogens.
See answer 1 Best Answer. The halogen are located in the group of seven A of the periodic table example of halogen are iodine and chlorine. How is the periodic law demonstrated within the groups of the periodic table.
How do the electron configurations within the same group of elements compare. According to Mendeleevs Periodic Law isotopes of an element must be given separate places in the periodic table because they have different atomic masses. The periodic law refers to the way in which the periodic table of elements is arranged.

Halogens In Aqueous Solution And Their Displacement Reactions Experiment Rsc Education

How Is The Periodic Law Demonstrated In Halogens Brainly Com
4 6 Looking For Patterns The Periodic Law And The Periodic Table Chemistry Libretexts
No comments for "How Is the Periodic Law Demonstrated in the Halogens"
Post a Comment