This Is a Net Gain or Loss of Electrons
This is a strong short-lived magnet created by a looped electrical current. 2s in Li 3s in Na 3p in Al etc they can be transferred to another atom which has orbitals that are lower energy eg.
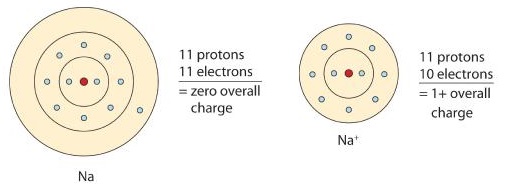
4 7 Ions Losing And Gaining Electrons Chemistry Libretexts
These reactions involve the oxidation or loss of electrons of one substance and the reduction or gain of electrons of another.
. This is a strong short-lived magnet created by a looped electrical current. Gaining an electron results in a negative charge so the atom is an anion. More penetrative than alpha.
If electrons are lost a cation is formed. This is a net gain or loss of electrons. Gain of electrons and loss of oxygen is called.
This is a net gain or loss of electrons. A reversible chemical reaction one reaction is of oxidation and the another of reduction. These reactions involve the oxidation or loss of electrons of one substance and the reduction or gain of electrons of another.
Net gain or loss of electrons. With the positive and negative charges canceling each other out a neutral atom has no net charge. Is there an overall gain or loss of electrons by an object when it is electrically polarized.
Energy created by the flow of electrons through a conductor. This is a flow of electrons that creates a charge one of the fundamental forms of energy. In some chemical reactions electrons are transferred from one substance to another but there is no net gain or loss of electrons during the reaction.
When an atom gainsloses an electron the atom becomes charged and is called an ion. Loss or gain of electrons BY A NEUTRAL atom results in the formation of an ion. Nfactor is a reaction specific parameter and for inter molecular redox reaction nfactor of oxidising reducing agent is the no.
This is a net gain or loss of electrons. In some chemical reactions electrons are transferred from one substance to another but there is no net gain or loss of electrons during the reaction. This problem has been solved.
This is a net gain or loss of electrons. See the answer See the answer See the answer done loading. As you know all electrons in atoms are placed in orbitals - what happens is that in case if orbitals on atom 1 are high in energy eg.
Of moles of electron gained loss by one mole of compound. This is a flow of electrons that creates a charge one of the fundamental forms of energy. This is a strong short-lived magnet created by a looped electrical current.
This type of reaction is called a redox reaction. This is a net gain or loss of electrons. Energy created by the flow of electrons through a conductor.
Here it says that when atoms lose bonds to hydrogen and gain bonds to other heteroatoms it is generally an oxidative process considering that H is the least electronegative. Losing an electron results in a positive charge so atom ion is a cation. This is a net gain or loss of electrons.
Oxidation loss of electrons Reduction gain of electrons Oxidation-reduction. Hope this helps Answer link. The refers to the functional form and parameter sets used to calculate the potential energy of a system of atoms or coarse-grained particles in molecular mechanics dynamics simulations.
An atom with a positive or negative charge has gained or lost electrons. Flow of electrons that creates a charge. Is there an overall gain or loss of electrons by an object when it is electrically polarized.
PARAGRAPH In a balance redox reaction net gain of electrons is equal to net loss of electronss. So when an alcohol gets converted to a ketone there is a net loss of electron density on the carbon atom - synonymous to the loss of electrons. This is what defines an ion the loss of gain of electrons to create a net charge.
No - if Iron is oxidised to Iron II or Iron III it LOSES electrons - but the oxygen or other gains electrons. Either the atom contains more protons than electrons or more electrons than protons resulting in the atom gaining a net charge. This type of reaction is called a redox reaction.
This type of reaction is called a redox reaction In neutral atoms such as A. In some chemical reactions electrons are transferred from one substance to another but there is no net gain or loss of electrons during the reaction. Now if you start thinking about which atoms have high and.
This is a flow of electrons that creates a charge one of the fundamental forms of energy. This is a net gain or loss of electrons. 2p in O and F 3p in Cl etc.
This is a strong short-lived magnet created by a looped electrical current. In some chemical reactions clectrons are transferred from one substance to another but there is no net gain or loss of electrons during the reaction. With the gain or loss of electrons the balance of protons and electrons is shifted.
50 ml 01M CUSO IS mixing 50 ml of 01M KI then number of moles of electron. If electrons are gained an anion is formed. So for the compound there is.
They involve the oxidation of one substance losing electrons and the reduction of another gaining. These reactions involve the oxidation or loss of electrons of one substance and the reduction or gain of electrons of another.

4 7 Ions Losing Gaining Electrons Youtube

No comments for "This Is a Net Gain or Loss of Electrons"
Post a Comment